Understanding the Interfacial Chemistry of Solvent Extraction
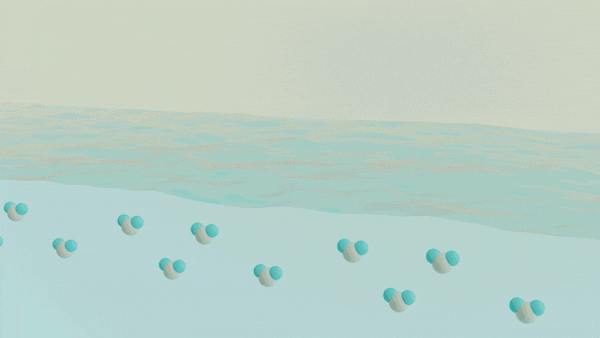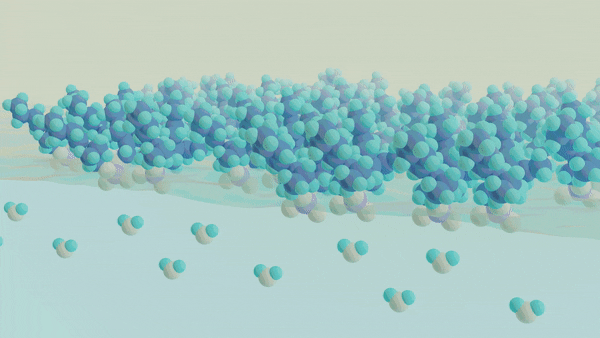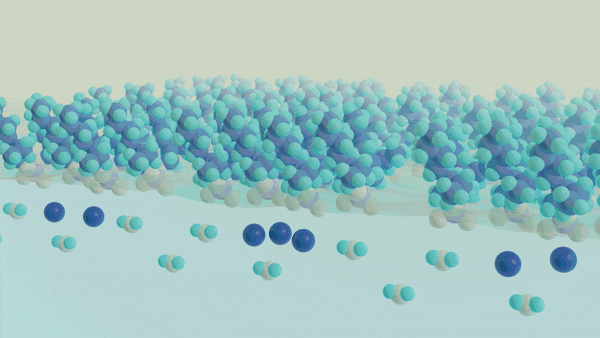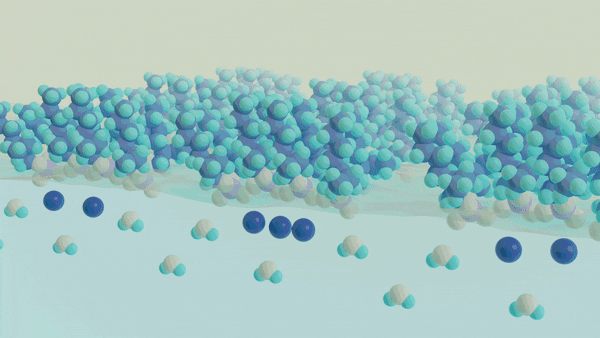
A major challenge in separating a mixture of molecules or ions from one another is understanding and controlling the chemical processes that occur at a liquid-liquid interface, such as oil and water. The interface between two liquid phases is a hotspot for molecular self-assembly and anomalous chemical processes that ultimately dictate how species can move from one phase to another. Despite the widespread use of liquid-liquid extraction methods, the chemical events governing selectivity and efficiency are not well understood, which limits our ability to rationally design new separations schemes to meet the demands of the next generation of clean energy technology. A key barrier to obtaining this insight is the current inability to directly probe the structure and dynamics of the molecularly thin interface using conventional spectroscopic methods while not being overwhelmed by the signals originating from the bulk phases. Thus, to understand the events at the interface, one must develop an approach that is uniquely capable of providing chemical insight into the interfacial chemical composition, organization, and dynamics. The overarching goal of this work is to develop a unified understanding of the molecular structure and dynamics governing the mechanisms of chemical separations at liquid-liquid interfaces using surface-specific spectroscopies, such as vibrational and electronic sum frequency generation, in conjunction with neutron scattering methods to advance the design of selective and efficient chemical separations.
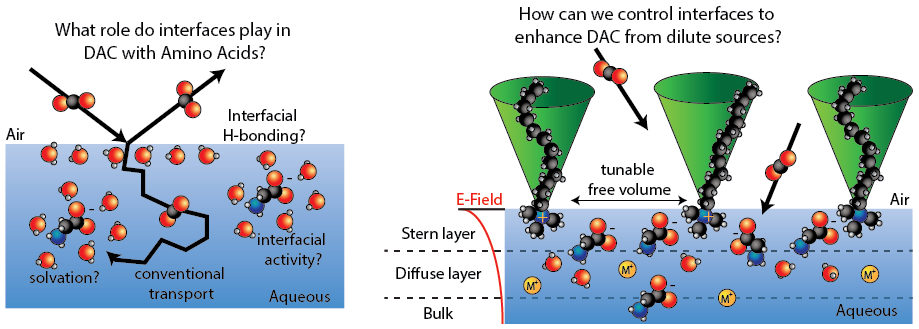
Controlling Surfaces for Efficient CO2 Direct Air Capture Chemistry
Closing the carbon cycle is one of the greatest scientific challenges of the 21st century. To decrease the CO2 levels in air to acceptable ranges, one needs to develop approaches to selectively bind CO2 at ~400 ppm levels in an efficient, cost-effective way. Although innovations for developing solutions to capture CO2 continue to grow, there are fundamental bottlenecks that prevent widespread implementation, in particular, the dubious role that interfaces play on mass transport and chemical reactivity. In this recently funded DOE-BES work, we seek to (1) understand molecular transport across interfaces in the context of direct air capture (DAC) of CO2 and (2) design new approaches to enhance reactivity and capture probabilities by controlling air/aqueous interfaces.
Selective Ligand Adsorption at Rare Earth Mineral Interfaces
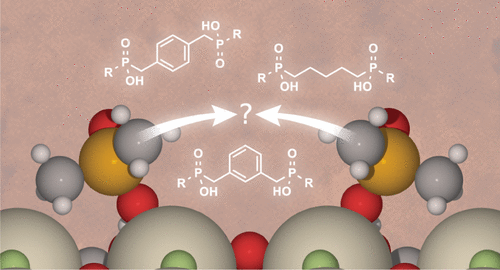
Rare earth elements (REE) are essential components in a diverse set efficient alternative energy and defense technologies. Despite their modest abundance in the Earth’s crust, they are so named because they are often present in mineral deposits at levels well below those economically accessible. As such, isolating the REs in pure forms represents a major concern in their continued use and in our current reliance on them. Ore beneficiation is often achieved through the process of froth flotation, wherein water and a collector molecule (surface active ligand) are added to crushed ores to selectively change surface hydrophobicity of a desired mineral that is skimmed off with air bubbles for further processing. Using current technology, an estimated 40% of the REEs go to tailings (waste), which highlights the need for more efficient strategies for extracting REEs from mined ore. Developing these new approaches, however, is limited by a relatively poor understanding of chemical selectivity at interfaces. In this work, we leverage surface specific spectroscopies supported by collaborators in theory and ligand design/synthesis to understand the structure and chemical makeup of complex ore-aqueous interfaces that leads to selective adsorption and enhanced beneficiation.
Non-Equilibrium Energy Transport in Catalysis
New project – info coming soon!
Abiotic-Biotic Material Interfaces
New project – info coming soon!
